1. VITA veneering and CAD/CAM materials
All VITA veneering and CAD/CAM materials bear a UDI-compliant Health International Bar Code (HIBC) that contains all the required information, either in digital form or in plain text. All units produced (e.g., bottles or blocks) are labeled with a batch number. The scannable barcode is provided on the primary packaging of the product.
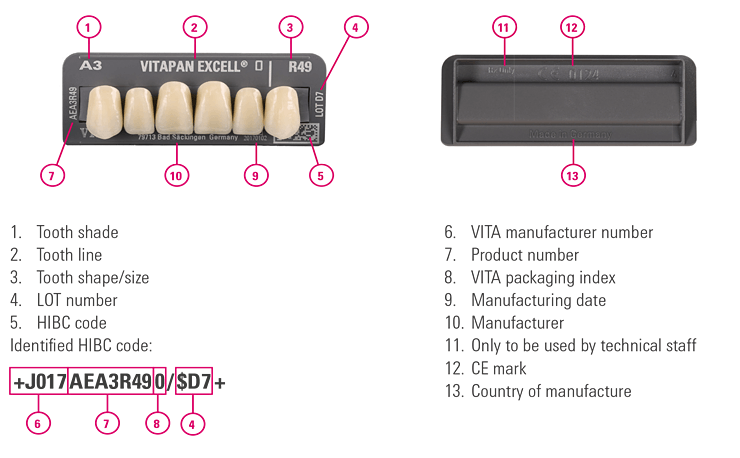
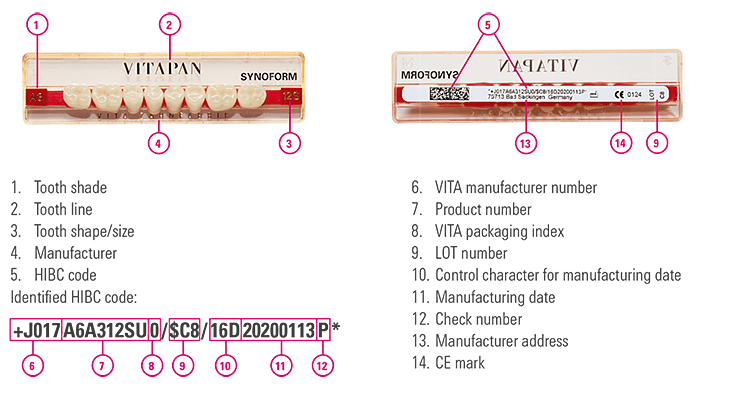
2. VITA prosthetic teeth
Our tooth sets are also labeled with a UDI-compliant Health International Barcode. Every tooth set includes a verified and approved tooth batch that ensures correct batch traceability. As the risk class is low, batch identification is not required for each individual tooth.